Unveiling the Mystery: The Evaporation Point of Alcohol
Ever wondered why that splash of wine disappears from your countertop or how a flambéed dessert ignites so dramatically? The answer lies in the evaporation of alcohol. Understanding the temperature at which alcohol evaporates unlocks a world of practical knowledge, from perfecting culinary techniques to optimizing cleaning solutions. Let's delve into the science behind this seemingly simple process and uncover its surprising applications.
The evaporation point of alcohol isn't a fixed number; it's a dance between molecular energy and atmospheric pressure. Pure ethanol, the type of alcohol found in alcoholic beverages, boils at approximately 173°F (78.3°C). However, various factors influence the actual temperature at which alcohol transitions from liquid to vapor. These include the alcohol's concentration, the surrounding temperature, and air circulation.
Historically, understanding the evaporation of alcohol has played a crucial role in the development of distillation techniques, dating back centuries. From ancient civilizations crafting spirits to modern-day perfumeries extracting fragrant essences, the controlled vaporization of alcohol has been instrumental. This knowledge has also been crucial in various industrial processes, from manufacturing pharmaceuticals to creating cleaning agents.
The significance of alcohol's evaporation point extends beyond historical applications. In cooking, it influences the flavor and texture of dishes. In cleaning, it determines the effectiveness of sanitizing solutions. Even in medicine, understanding alcohol evaporation is vital for sterilizing equipment and preparing tinctures.
A key issue related to alcohol evaporation is its flammability. The vapor produced when alcohol evaporates is highly flammable, necessitating caution when working with alcohol near open flames or heat sources. Understanding the factors influencing evaporation rates, such as concentration and temperature, can help mitigate potential risks.
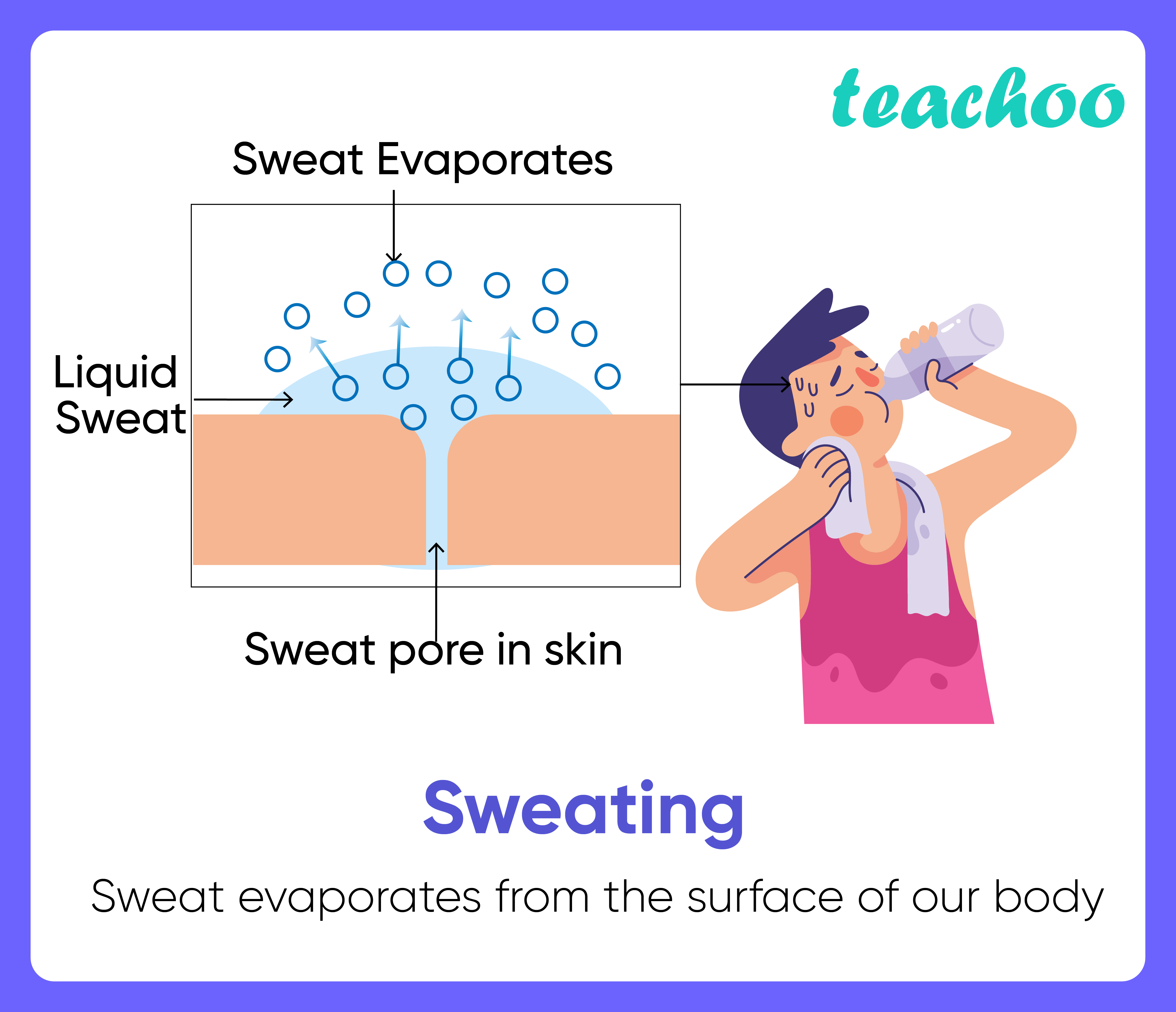
Simply put, evaporation is the process by which a liquid transforms into a gas. For alcohol, this occurs when the molecules gain enough energy to overcome the forces holding them together in liquid form. The higher the temperature, the faster the molecules move, and the more readily they escape into the air as vapor. For example, a spill of vodka (around 40% alcohol) will evaporate more slowly than pure ethanol due to the presence of water.
One benefit of understanding alcohol evaporation is in culinary arts. Chefs use this knowledge to control the intensity of alcohol flavors in sauces and desserts. Another advantage lies in cleaning and disinfection. The rapid evaporation of alcohol-based cleaners contributes to their effectiveness in eliminating germs and bacteria. Lastly, in the production of perfumes, the evaporation rate of alcohol plays a vital role in the fragrance's longevity and how its scent unfolds over time.
When working with alcohol, especially near heat sources, ensure proper ventilation to disperse flammable vapors. Store alcohol in tightly sealed containers away from direct sunlight and heat. When cooking with alcohol, never add it directly to a hot pan; instead, allow the pan to cool slightly before incorporating the alcohol.
Advantages and Disadvantages of Understanding Alcohol Evaporation
| Advantages | Disadvantages |
|---|---|
| Improved culinary skills | Risk of fire if not handled carefully |
| Enhanced cleaning effectiveness | Potential for inhalation hazards in confined spaces |
| Better understanding of perfume composition | Can damage certain materials if used improperly |
Five real-world examples of alcohol evaporation include: the creation of hand sanitizers, the production of alcoholic beverages, the process of flambéing in cooking, the use of alcohol-based markers, and the application of alcohol-based perfumes.
Frequently Asked Questions:
1. Does alcohol evaporate at room temperature? Yes, although the rate of evaporation is slower than at higher temperatures.
2. Does the type of alcohol affect its evaporation rate? Yes, different types of alcohol have different boiling points, influencing their evaporation rates.
3. Is it safe to cook with alcohol? Yes, as long as precautions are taken, such as avoiding open flames and ensuring proper ventilation.
4. Why does rubbing alcohol feel cold on the skin? The evaporation of alcohol absorbs heat from the skin, creating a cooling sensation.
5. How does humidity affect alcohol evaporation? Higher humidity slows down the rate of evaporation.
6. Can alcohol evaporate completely from a sealed container? No, in a sealed container, evaporation will reach an equilibrium where the rate of evaporation equals the rate of condensation.
7. Does the surface area affect alcohol evaporation? Yes, a larger surface area leads to faster evaporation.
8. What is the flash point of alcohol? The flash point is the lowest temperature at which alcohol can vaporize to form an ignitable mixture in air.
One tip for working with alcohol is to use a thermometer to monitor the temperature, particularly when cooking or distilling. This can help ensure the process is controlled and safe. Another trick is to use a fan to increase air circulation, which can accelerate the rate of evaporation.
In conclusion, understanding the temperature at which alcohol evaporates and the factors influencing this process has far-reaching implications. From enhancing culinary creations to optimizing cleaning solutions and even informing safety practices, this knowledge proves valuable in diverse areas. By grasping the science behind alcohol evaporation, we can harness its power effectively and safely. The ability to manipulate and control this process empowers us to create, innovate, and understand the world around us with a deeper appreciation for the seemingly simple yet fascinating phenomenon of evaporation. Remember to always prioritize safety when working with alcohol, especially near heat sources, and never underestimate the power of this volatile liquid. Continue exploring the science behind everyday phenomena; you never know what wonders you might uncover. By paying attention to these details, we can ensure both effective use and personal safety.
Dish network fireplace channel
Understanding non itchy red blotches on arms
Red aesthetic collage wallpaper laptop grunge a bold statement

1 Why does water evaporate at room temperature | Solidarios Con Garzon

How Does Water Evaporate At Night at Rita Guerra blog | Solidarios Con Garzon

Illustrated Glossary of Organic Chemistry | Solidarios Con Garzon

How To Evaporate Alcohol From Tinctures | Solidarios Con Garzon

Why Does Oil Not Evaporate | Solidarios Con Garzon
Vaporization Examples Real Life | Solidarios Con Garzon

Can Perfume Evaporate Everything You Need To Know 2024 | Solidarios Con Garzon

How Fast Does Alcohol Evaporate At Room Temperature and When Boiling | Solidarios Con Garzon

What Evaporates Quicker Water Or Alcohol at Dolores Boren blog | Solidarios Con Garzon

Why Does Water Evaporate At Room Temperature | Solidarios Con Garzon

Why Does Water Evaporate At Room Temperature | Solidarios Con Garzon

at what temperature does alcohol evaporate | Solidarios Con Garzon

Why Does Water Evaporate At Room Temperature | Solidarios Con Garzon

How Fast Does Alcohol Evaporate At Room Temperature and When Boiling | Solidarios Con Garzon

Cannabis helps my Crohns | Solidarios Con Garzon