Unlocking Chemical Secrets with Potential Energy Diagrams
Ever wondered how chemists predict the outcome of a reaction? Or how they understand the energetic dance of molecules as they transform? The answer lies in a powerful tool: the potential energy diagram. These diagrams aren't just static illustrations; they're dynamic maps of chemical reactions, revealing the energy changes that drive transformations.
Potential energy diagrams chart the energy landscape of a chemical reaction. They show how the energy of a system changes as reactants morph into products. Imagine climbing a hill – that's analogous to the energy input required for some reactions to proceed. The peak of the hill represents the transition state, a fleeting configuration where old bonds break and new ones begin to form.
The concept of charting energy changes in chemical reactions has roots in the late 19th and early 20th centuries, evolving alongside advancements in thermodynamics and kinetics. Scientists like Svante Arrhenius contributed significantly to our understanding of reaction rates and the role of energy barriers. Today, potential energy diagrams are essential in various branches of chemistry, from organic synthesis to materials science.
These diagrams are crucial for understanding reaction mechanisms, predicting reaction rates, and designing catalysts. They help us grasp the energetic feasibility of a reaction, revealing whether a reaction will occur spontaneously or require an energy boost. They also show the difference in energy between reactants and products, providing insights into the stability of these species.
A fundamental aspect of these diagrams is the activation energy, the minimum energy required to initiate a reaction. Think of it as the "push" needed to get a reaction going. The lower the activation energy, the faster the reaction. Catalysts work by lowering this activation energy, accelerating reactions without being consumed themselves.
One benefit of using these diagrams is the ability to visualize reaction pathways. This allows chemists to identify intermediates, which are temporary species formed during the reaction process. Understanding these intermediates can be key to controlling the outcome of a reaction. For example, in a multi-step reaction, the diagram can reveal which step is the rate-determining step, the slowest step that dictates the overall reaction rate.
Another advantage is the ability to predict the spontaneity of a reaction. If the products are lower in energy than the reactants, the reaction is exergonic (releases energy) and tends to be spontaneous. Conversely, if the products are higher in energy, the reaction is endergonic (requires energy input) and is not spontaneous under standard conditions.
Furthermore, these diagrams can help predict the effect of temperature on reaction rates. Higher temperatures provide molecules with more energy, increasing the likelihood they will overcome the activation energy barrier and react.
To interpret a potential energy diagram, look at the relative energies of reactants, products, and the transition state. The difference in energy between reactants and products represents the enthalpy change of the reaction. The difference between reactants and the transition state is the activation energy.
A simple example is the reaction of hydrogen and oxygen to form water. The diagram would show reactants (H2 and O2) at a certain energy level, a transition state at a higher energy level, and products (H2O) at a lower energy level, indicating an exothermic reaction.
Here are a few tips for understanding these diagrams. Firstly, remember that the x-axis represents the reaction progress, not time. Secondly, the y-axis represents potential energy. Finally, the higher the peak of the transition state, the higher the activation energy and the slower the reaction.
Frequently asked questions about potential energy diagrams include: What is activation energy? How do catalysts affect the diagram? How can we predict reaction spontaneity? What is the significance of the transition state? What does the shape of the curve tell us? How do temperature and concentration influence the reaction? What are the limitations of these diagrams? These questions are commonly addressed in chemistry courses.
In conclusion, potential energy diagrams are essential tools in chemistry, providing a visual representation of the energetic landscape of chemical reactions. They unlock crucial information about reaction mechanisms, reaction rates, and spontaneity. By understanding these diagrams, we gain deeper insights into how chemical transformations occur and how to control them. These concepts are fundamental to advanced studies in chemistry and related fields, enabling scientists to design new materials, develop efficient catalysts, and understand complex biological processes. Explore further resources like textbooks, online tutorials, and educational software to delve deeper into this fascinating area of chemistry. Understanding these diagrams is not just about passing chemistry exams; it's about unraveling the secrets of the molecular world around us.
Praying time lyrics a moment of reflection
Unleash creativity a parents guide to the amazon kids drawing tablet
Broken gate bay area gate repair solutions

Potential Energy Diagram Explained | Solidarios Con Garzon

How To Read Energy Diagrams Chemistry | Solidarios Con Garzon
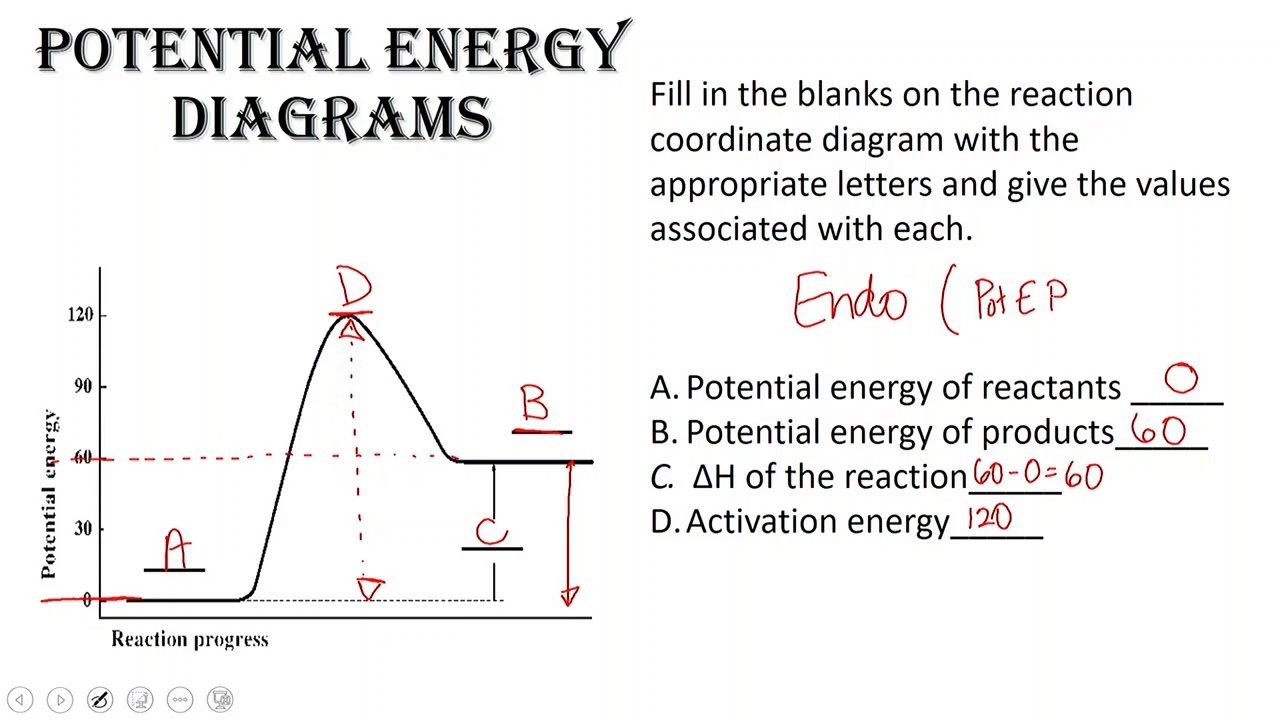
How To Read An Energy Diagram | Solidarios Con Garzon

How To Read Potential Energy Diagram | Solidarios Con Garzon
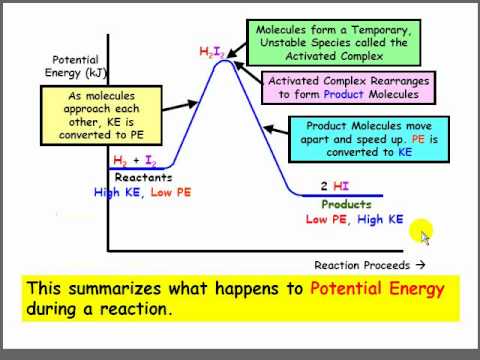
Potential Energy Diagram Chemistry | Solidarios Con Garzon

How To Read Energy Diagrams Chemistry | Solidarios Con Garzon

How To Read Potential Energy Diagram | Solidarios Con Garzon

potential energy diagrams chemistry | Solidarios Con Garzon

Identifying Endothermic And Exothermic Reactions Worksheet | Solidarios Con Garzon
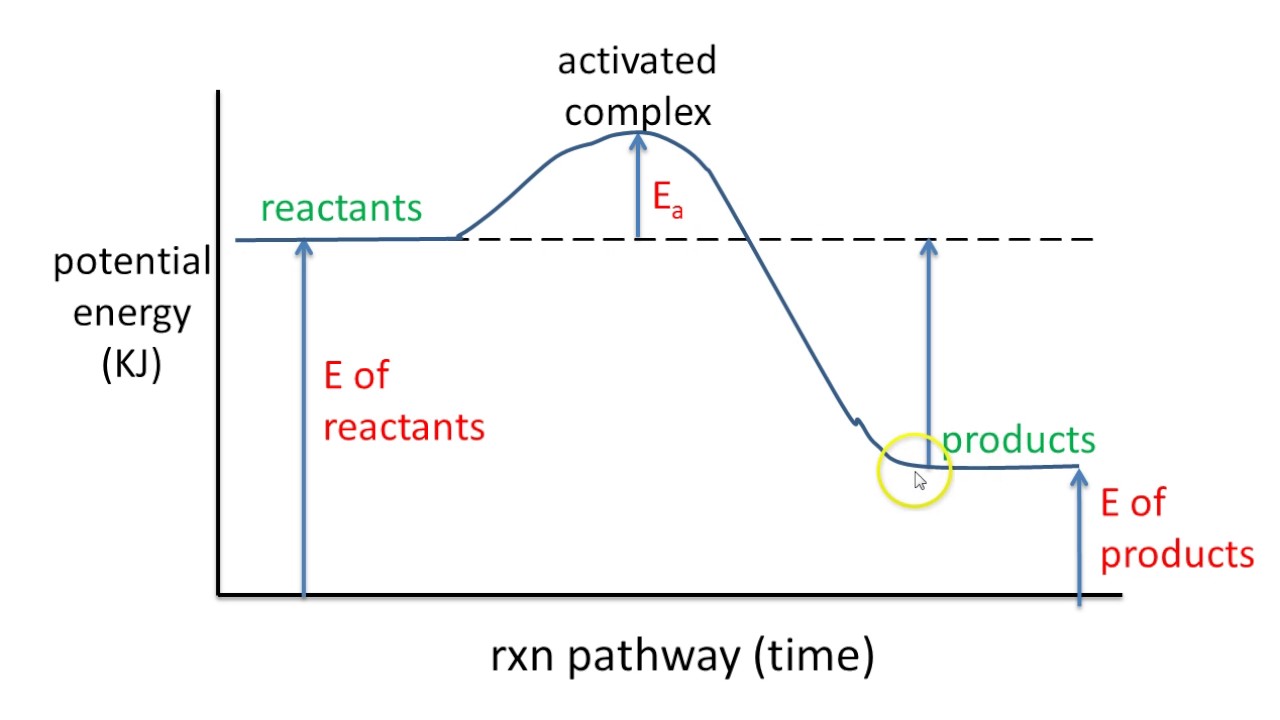
Potential Energy Diagram Exothermic Reaction | Solidarios Con Garzon

Potential Energy Diagram Labeled | Solidarios Con Garzon

Endothermic Potential Energy Diagram | Solidarios Con Garzon

Chemistry 12 Worksheet 1 2 Potential Energy Diagrams Answers | Solidarios Con Garzon

How To Read Energy Diagrams Chemistry | Solidarios Con Garzon

Potential Energy Diagram Physics | Solidarios Con Garzon